Pharmaceutical use and water integration system
Price 10000.0 USD ($)/ Unit
Pharmaceutical use and water integration system Specification
- Installation
- Turnkey supply and installation
- Flow Rate Range
- From 500 LPH to 20,000 LPH
- Maintenance
- Low maintenance with automated monitoring
- Power Supply
- 380V/50Hz, customizable on request
- Control System
- Digital with touch screen interface
- Output Water Quality
- Meets USP, EP, and JP pharmaceutical standards
- Operating Temperature
- 5°C - 45°C
- Filtration Level
- Multi-stage filtration including pre-filter, RO, and UV sterilization
- System Integration
- PLC controlled, SCADA compatible
- Piping Material
- SS316L with electropolished finish
- Sanitization Method
- Hot water or chemical sanitization options
Pharmaceutical use and water integration system Trade Information
- Minimum Order Quantity
- 1 Unit
- Payment Terms
- Telegraphic Transfer (T/T), Letter of Credit (L/C)
- Supply Ability
- 1 Unit Per Week
- Delivery Time
- 3 Months
- Sample Available
- No
- Main Export Market(s)
- Asia, Australia, Central America, North America, South America, Eastern Europe, Western Europe, Middle East, Africa
- Main Domestic Market
- All India
- Certifications
- FDA/cGMA
About Pharmaceutical use and water integration system
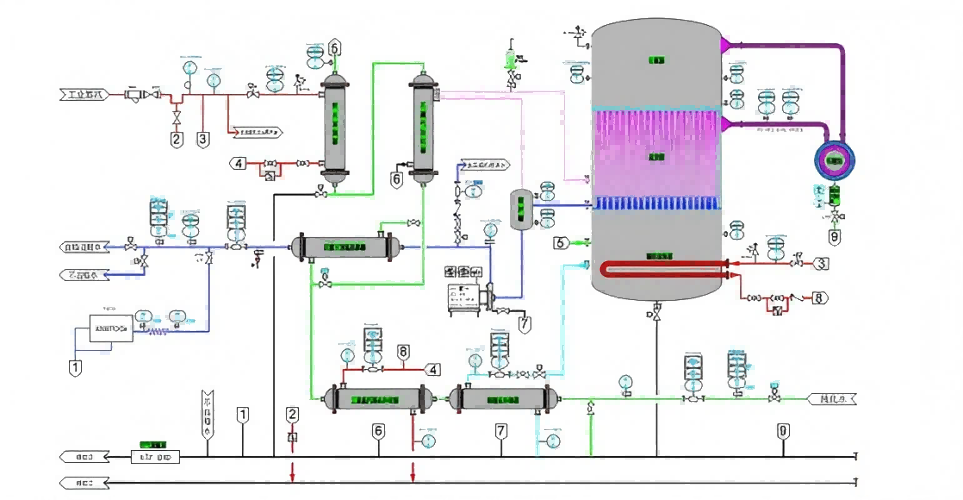
1.Pre-processing unit
The modular pretreatment unit is designed according to the inlet characteristics. The robust design is designed to pretreatment the water according to the final treatment requirements. Provide stable performance and ensure the maximum availability of the system.
2.Pure water and water for injection
Pure water (PW) or water for injection (WFI) is produced for all pharmacopoeia, generating PW or WFI using the core technology of reverse osmosis and continuous electric DI. Water for injection is generated by a secondary membrane system with increased ultrafiltration function and does not go through a distillation process.
3.Store and distribute the modules
Standard distribution functions can be implemented in a single module and deliver the quality and quantity of the customer. The distribution system solution integrates all the technical convergence, instrument control in one system, and maximizes the need for space. The overall system design provides reliable operation, continuous monitoring, while maintaining optimal energy efficiency and reducing operating costs without compromising quality.
4.Pure steam generator
The steam generator can provide the dry saturated steam conforming to the Pharmacopoeia specifications, the vertical design of the pure steam generator has the DTS evaporator, and the separator ensures that no microdroplets or impurities pass through the product steam. The generator is designed to have an energy recovery and a non-condensing gas evaporation system to provide a constant high-purity steam.
5.Vertical hot-pressed distilled water machine
The vertical hot press was designed and manufactured in full accordance with cGMP guidelines, FDA and EMEA requirements for the production of injection water (sterile water without heat source). The key thrust of this system is the efficiency of energy conservation to ensuring the maximization of energy utilization without affecting quality.
6.Integrate the automation systems
The whole process can be monitored and controlled to control the output quality parameters and grasp the performance information.
7.Strict material technology
316L / 304 stainless steel meeting the material and process requirements, ASME BPE or ISO20037.
Multi-Stage Filtration for Pharmaceutical Purity
The system employs a combination of pre-filter, reverse osmosis, and UV sterilization, ensuring consistently high water purity. This advanced setup meets or exceeds the stringent requirements outlined by USP, EP, and JP pharmaceutical standards. Each filtration stage is meticulously integrated to eliminate particulates, dissolved solids, and microbial contaminants, safeguarding your pharmaceutical processes from water-borne risks.
Advanced Integration and Control
Designed for modern pharmaceutical environments, the system features PLC-controlled automation and SCADA compatibility for comprehensive oversight. The intuitive touch screen interface streamlines operation, monitoring, and troubleshooting. This digital control infrastructure supports efficient process management, easy customization, and immediate responses to performance or quality deviations, optimizing overall reliability and productivity.
Turnkey Installation and Robust Support
From supply to installation, our turnkey solution covers every aspect, ensuring a hassle-free setup tailored to your facilitys needs. The use of premium SS316L piping with an electropolished finish guarantees high durability and hygiene. Automated monitoring and low-maintenance design reduce downtime, while our expert support infrastructure in India ensures ongoing technical assistance and smooth operation for global clients.
FAQs of Pharmaceutical use and water integration system:
Q: How does the multi-stage filtration ensure pharmaceutical-grade water quality?
A: The system combines pre-filtration, reverse osmosis, and UV sterilization to eliminate physical impurities, reduce dissolved solids, and neutralize microorganisms. This thorough approach guarantees water meets USP, EP, and JP pharmaceutical standards, making it suitable for sensitive pharmaceutical processes.Q: What are the benefits of PLC control and SCADA compatibility in this water integration system?
A: PLC control allows for reliable automation and precise management of purification processes, while SCADA compatibility facilitates real-time data monitoring and remote control. This integration results in greater process efficiency, enhanced traceability, and easier troubleshooting for pharmaceutical environments.Q: When should the hot water or chemical sanitization options be used?
A: Sanitization should be performed based on a scheduled maintenance protocol or whenever microbiological contamination risks are detected. Hot water is ideal for routine thermal disinfection, while chemical sanitization can target specific contaminants. Both methods support compliance with strict pharmaceutical hygiene standards.Q: Where can this system be installed, and are there location-specific adjustments available?
A: The system is suitable for pharmaceutical manufacturing facilities worldwide. Power supply and certain specifications, such as voltage, can be customized during installation to accommodate local requirements. Our turnkey services and international expertise ensure seamless implementation wherever needed.Q: What maintenance processes are required for this integrated water system?
A: Regular automated monitoring significantly reduces manual maintenance. Routine tasks include system inspections, filter replacement, and periodic sanitization. The digital interface provides alerts and diagnostic information, ensuring timely interventions and minimizing operational downtime.Q: How can pharmaceutical facilities use this system to improve operational efficiency?
A: By providing consistent water quality and automated control, the system minimizes manual intervention and batch rejections. PLC and SCADA integration enable centralized management, while low-maintenance design reduces disruptions, contributing to smoother workflows and cost savings.

Price:
- 50
- 100
- 200
- 250
- 500
- 1000+
More Products in Water Treatment Technology Category
Production process and technology of distilled water and pure water
Price 10000.0 USD ($) / Unit
Minimum Order Quantity : 1 Unit
Diffusion dialysis - waste acid recovery technology
Price 1000.0 USD ($) / Plant
Minimum Order Quantity : 1 Plant
Diffusion dialysis and electrodialysis combined technology
Price 1000.0 USD ($)
Minimum Order Quantity : 1

 Send Inquiry
Send Inquiry





 Send Inquiry
Send Inquiry